- What is amniomatrix +
- BENEFITS +
- Indications +
- Collection, Packaging, and Storage +
- Available sizes +
- Application +
- Reimbursement and Billing +
- Downloads +
- REFERENCES +
AmnioMatrix is an amniotic membrane graft which brings a new generation of ocular surface tissue therapy to South Africa. AmnioMatrix can accelerate the regeneration of damaged tissue and reduce discomfort and pain for the patient, associated with the reconstruction of the ocular surface.
The amniotic membrane is the innermost membrane of the placenta and it contains natural growth factors and cytokines that are integral to the development of a healthy fetus. When applied to the ocular surface, the amniotic membrane promotes wound healing and may assist with pain reduction at the affected site. The amniotic membrane allografts are thin, opaque and extremely lightweight.
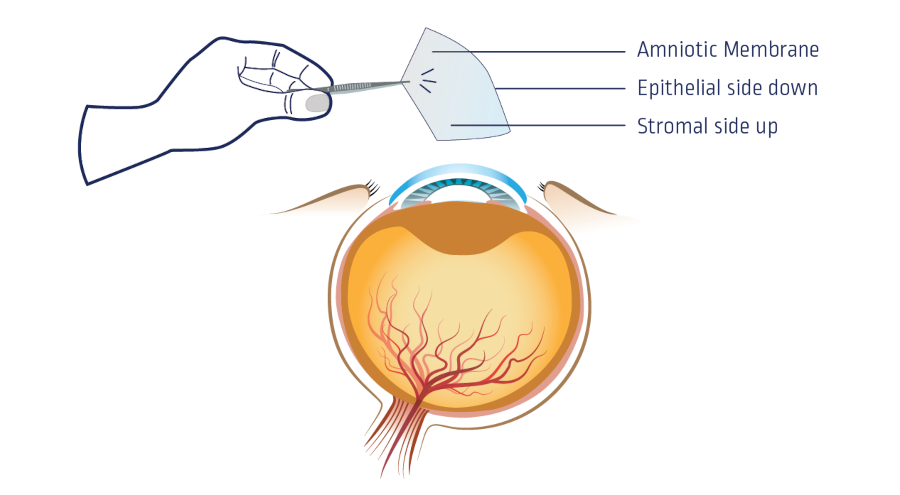
The amniotic membrane is used for its high concentration of cytokines and growth factors, and acts as a potent facilitator in wound healing. Generally, the membrane is placed with the epithelial side down in contact with the wound surface in order to efficiently release the growth factors to the wound site.
The use of the amniotic membrane to cover inflamed or exposed areas favorably influences the wound healing process, as well as, reduces the levels of discomfort for the patient. The amniotic membrane conforms easily to the ocular surface and can be either glued or sutured to the ocular surface. The membrane is hydrophilic and naturally absorbs the surrounding fluids. As part of the healing process the amnion resorbs into the wound. General literature suggests that the membranes completely resorb into the wound in about 4-6 weeks1.

Cryopreserved
- Fresh amniotic membrane that is processed and stored frozen at -80°C
- The epithelial layer of cells is retained as well as the important biological factors and cellular mediators
- Supports epithelial healing and serves as a physical barrier for the ocular surface against the external environment
Dehydrated
- Dry amniotic membrane that has had the epithelial layer of cells removed
- No living cells are exposed to the patient
- Membrane acts as a scaffold onto which new cells can grow once it has been placed on the wound
- Provides natural healing properties to wounds with minimal inflammation and scarring
The benefits of AmnioMatrix include:
- Provides a structure for cellular migration and proliferation
- Contains collagen types IV, V and VII which promote cellular differentiation and adhesion
- Known to be antiangiogenic
- Anti-inflammatory effect
- Bacteriostatic effect
- Anti-adhesive and supportive in preventing scarring
- Assists with pain and discomfort at affected site
- Known to be non-immunogenic and low in antigenicity
- Provision of a natural biological barrier
Amniotic membrane, both dehydrated and cryopreserved, is used as a substrate to replace the damaged ocular or subcutaneous tissue or as a biological dressing in:
|
Indications of Corneal Surface Reconstruction |
Indications of Conjunctival Surface Reconstruction |
Contraindications
|
|
|
Amniotic Membrane should NOT be implanted into:
|
Collection
Placental donations are collected during the birthing process in accordance the National Health Act of South Africa. All donors complete a health questionnaire and are screened for transmissible diseases. All AmnmioMatrix samples are tested during processing for bacterial and fungal contamination and to ensure sterility of the product.
Packaging
|
Dehydrated |
Cryopreserved |
|
The amniotic membrane is spread on a polyester net with the epithelial side orientated onto the net. The membrane is packaged aseptically in an inner polyethylene pouch and sealed with an outer peel pouch. Dehydrated denuded human amniotic membrane is terminally sterilised by gamma-irradiation. |
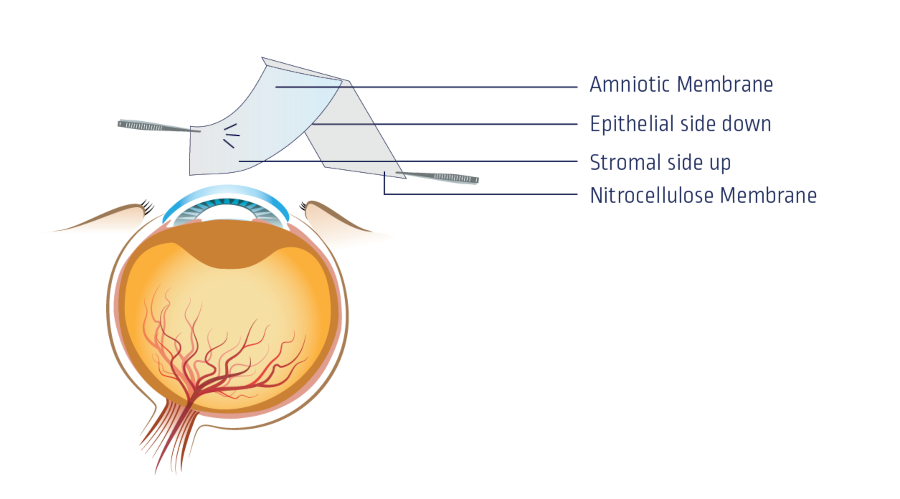
The amniotic membrane is processed in sterile conditions and decontaminated by antibiotic incubation. The membrane is spread over on nitrocellulose membrane with the stromal side of the amnion against the nitrocellulose membrane. Thus, the epithelial side is away from the nitrocellulose membrane.
The membrane is then placed in a polyethylene pouch with Dulbecco’s Modified Eagle Medium and Glycerol as storage media for protection against freeze injury. The membranes are cryopreserved at -80°C. |
Storage
|
Dehydrated |
Cryopreserved |
|
Dehydrated AmnioMatrix can be stored in a clean dry setting at room temperature for up to 4 years from date of manufacture. No special storage is needed for this product. |
Cryopreserved AmnioMatrix is shipped frozen on dry ice. Once received, AmnioMatrix may be placed in a home freezer (-20°C) for up to 3 months or until expiration date printed on outer product packaging, whichever comes first. |
HCP-AmnioMatric- -Application Techniques
Application Techniques:
A. Inlay (Graft)
- The amniotic membrane is usually placed epithelial side up.
- Epithelium grows over the graft, securing itself to the basement membrane.
- The amniotic membrane is intended to act as a substrate or scaffold for epithelial cells to grow and is therefore incorporated into the host tissue (cornea or conjunctiva).
B. Overlay (Patch)
- Amniotic membrane is usually placed epithelial side down.
- Shallow corneal defects.
- Epithelium grows beneath the patch, which dissolves within a few weeks.
- Here the amniotic membrane functions essentially as a cover or a biological bandage ‘contact lens,’ protecting the underlying healing epithelial surface.
- The intention is for the membrane to fall off or be removed over a period of time.
C. Sandwich
- Support for deeper corneal ulcers.
- Fill the defect with layers of amniotic membrane, epithelial side up.
- Single sheet to secure the multiple layers over the top.
- Top layer can be epithelial side up or down.
How to bill accounts for amniotic membrane procedures:
Step 1: Procedure code + Diagnostic code (ICD10)
Step 2: 0201 (Material stock code) and NAPPI Code + Diagnostic code (ICD10) + Authorisation Code
|
Product Code |
Description |
Size |
NAPPI Code |
|
LAM1515 |
Dehydrated Membrane |
1.5x1.5cm |
493575001 |
|
LAM2020 |
Dehydrated Membrane |
2.0x2.0cm |
493581001 |
|
LAM3030 |
Dehydrated Membrane |
3.0x3.0cm |
493583001 |
|
LAM4040 |
Dehydrated Membrane |
4.0x4.0cm |
493584001 |
|
CAM2020 |
Cryopreserved Membrane |
2.0x2.0cm |
651936001 |
|
CAM4040 |
Cryopreserved Membrane |
4.0x4.0cm |
651938001 |
|
CAM6060 |
Cryopreserved Membrane |
6.0x6.0cm |
165595001 |
|
Procedure Codes |
Description |
ICD-10 Diagnosis Code |
|
3191 |
Blepharoplasty |
H02.03 |
|
3163 |
Excision Papilloma |
D23.1 |
|
3181, 0201 |
Ectropion/Entropion repair |
H02.1/0 |
|
3121, 0201 |
Corneal transplant |
H18.6 |
|
3120, 3132, 3198, 3126, 0201 |
Lasik POI |
H52.0/1/2 |
|
3125 |
Pterygium |
H11.0 |
|
3134 |
Pterygium/conjunctival cyst/ conjunctival tumour with graft used |
H11.0 |
For additional information on ICD-10 Codes, follow the link: ICD-10 Master Industry Table – National Department of Health
- Dua HS, Gomes JA., King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Survey of Ophthalmology. 2004 Jan;49(1):51–77.
- Ahn J-I, Jang I-K, Lee D-H, Seo Y-K, Yoon H-H, Shin Y-H, et al. A comparison of lyophilized amniotic membrane with cryopreserved amniotic membrane for the reconstruction of rabbit corneal epithelium. Biotechnology and Bioprocess Engineering. 2005;10(3):262–9.
- Baradaran-Rafii A, Aghayan H-R, Arjmand B, Javadi M-A. Amniotic membrane transplantation. Journal of Ophthalmic & Vision Research. 2008;2(1):58–75.
- Hao Y, Ma DH-K, Hwang DG, Kim W-S, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–52.
- Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20(4):408–13.
- Riau AK, Beuerman RW, Lim LS, Mehta JS. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010 Jan;31(2):216–25.
- Letko E, Stechschulte SU, Kenyon KR, et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch Ophthalmol. 2001;119(5):659-663.
- Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85(5):567-575.
- Pires RT, Tseng SC, Prabhasawat P, et al. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999;117(10):1291-1297.
- Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110(8):1585-1592.
- Anderson DF, Prabhasawat P, Alfonso E, Tseng SC. Amniotic membrane transplantation after the primary surgical management of band keratopathy. Cornea. 2001;20(4):354-361.
- Solomon A, Meller D, Prabhasawat P, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109(4):694-703.
- Shanbhag SS, Rashad R, Chodosh J, Saeed HN. Long-Term Effect of a Treatment Protocol for Acute Ocular Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Am J Ophthalmol. 2019;208:331-341.
- Arora R, Mehta D, Jain V. Amniotic membrane transplantation in acute chemical burns. Eye (Lond). 2005;19(3):273-278.
- Bissen-Miyajima H, Monden Y, Shimazaki J, Tsubota K. Cataract surgery combined with ocular surface reconstruction in patients with severe cicatricial keratoconjunctivitis. J Cataract Refract Surg. 2002;28(8):1379-1385.
- Palamar M, Kaya E, Egrilmez S, Akalin T, Yagci A. Amniotic membrane transplantation in surgical management of ocular surface squamous neoplasias: long-term results. Eye (Lond). 2014;28(9):1131-1135.
- Nagai-Kusuhara A, Nakamura M, Fujioka M, Negi A. Long-term results of amniotic membrane transplantation-assisted bleb revision for leaking blebs. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):567-571.
- Sarnicola V, Millacci C, Toro Ibanez P, Sarnicola C, Sarnicola E, Ruggiero A. Amniotic membrane transplantation in failed trabeculectomy. J Glaucoma. 2015;24(2):154-160.
- Honavar SG, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction in Stevens-Johnson syndrome. Ophthalmology. 2000;107(5):975-979.
- Thatte S, Jain J. Fornix Reconstruction with Amniotic Membrane Transplantation: A Cosmetic Remedy for Blind Patients. J Ophthalmic Vis Res. 2016;11(2):193-197.
- Slentz DH, Joseph SS, Nelson CC. The Use of Umbilical Amnion for Conjunctival Socket, Fornix, and Eyelid Margin Reconstruction. Ophthalmic Plast Reconstr Surg. 2020;36(4):365-371.
- Ti SE, Tow SL, Chee SP. Amniotic membrane transplantation in entropion surgery. Ophthalmology. 2001;108(7):1209-1217.
- Casas VE, Kheirkhah A, Blanco G, Tseng SC. Surgical approach for scleral ischemia and melt. Cornea. 2008;27(2):196-201.
- Pan X, Zhang D, Jia Z, Chen Z, Su Y. Comparison of hyperdry amniotic membrane transplantation and conjunctival autografting for primary pterygium. BMC Ophthalmol. 2018;18(1):119.
Next Biosciences
Ariane Avenue
International Business Gateway
Cnr. New Road and 6th Road
Midrand, South Africa
Office Hours
Monday - Thursday: 08h00 - 17h00
Friday: 08h00 - 16h00