Chromosomal causes of miscarriages
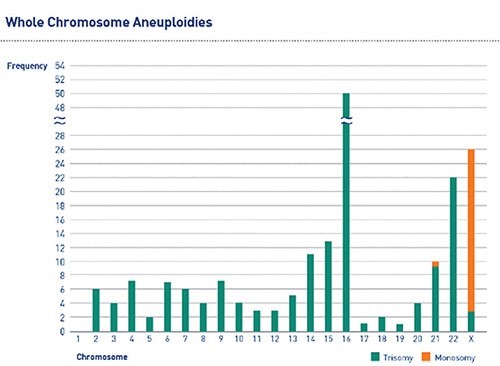
About 10-15% of all clinically recognised pregnancies result in spontaneous miscarriage1. Chromosomal aneuploidies are the most common cause of first-trimester miscarriage, accounting for approximately 50% of spontaneous abortions2. Aneuploidies observed in products of conception (POCs) involve almost every chromosome (Figure 1), with trisomies being the most common chromosomal abnormality3.
Figure 1: The frequency of chromosomal aneuploidies
Technologies for genetic testing of POCs
Karyotyping is a conventional method for chromosome analysis of POCs. While karyotyping plays an important role in investigating the reason for miscarriage, the technique is hindered by high failure rates (due to cell culture failures) and maternal cell contamination. Molecular approaches to quantify chromosomes, such as fluorescent in situ hybridisation (FISH) and quantitative polymerase chain reaction (qPCR), overcome some disadvantages of karyotyping but are also limited as they are restricted to coverage of only a subset of chromosomes, which may only account for up to approximately 58% of aneuploidies (and hence miss up to 42% of aneuploidies)3. Molecular karyotyping techniques using array comparative genomic hybridisation (aCGH) or next-generation sequencing (NGS) overcome limitations of other technologies by offering comprehensive chromosome screening (screening Chromosomes 1-22, X&Y) and do not require cell culturing (mitigating test failures).
The value of testing POCs
Considering the high incidence of genetic factors causing miscarriage, there is value in testing POCs following an early pregnancy loss. The majority of miscarriages are a result of sporadic chromosomal abnormalities, and the risk of recurrence of such foetal aneuploidies decreases in subsequent pregnancies4. Although many chromosomal abnormalities that cause miscarriages are sporadic and have a low recurrence risk, some abnormalities (such as translocations) are expected to significantly increase the risk of recurrence – which may necessitate parental karyotyping.
By identifying the approximately 50% of women whose pregnancy loss was due to chromosomal abnormalities, comprehensive chromosome screening will prevent a large proportion of patients from undertaking unnecessary and costly evaluations5. Furthermore, the psychological benefit of identifying the aetiology of a fetal loss cannot be understated5. Conversely, if a foetal chromosomal abnormality is excluded, there may be a possible treatable physiological cause for a given miscarriage, and investigations can be focussed on identifying this. Genetic testing outcomes can therefore be used to guide counselling for future pregnancies.
Genesis Genetics has recently introduced Prenatal Aneuploidy Screening (PAS), comprehensive chromosome screening of POCs.
References
Rai and Regan (2006) Recurrent miscarriage. Lancet 368:601-611
Van den Berg et al (2012) Biochem Biophys Acta. 1822:1951-1959
Shen et al (2016) Molecular cytogenetics. 9:7
RCOG https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg17/
Sahoo et al (2017) Genetics in Medicine. 19(1):83-89

 Back to Blogs
Back to Blogs