A dry eye disease decision tree
With the DEWS guidelines almost ten years old, new approaches are needed.
I am sometimes asked about medical decision-making by students, interns, fellows, or optometry colleagues. “How did you come up with that decision in managing that ocular surface disease case?” I give the answer appropriate to the particular case, but I also note that sometimes it comes down to experience. “That’s why they call it ‘practising medicine,’ I say. “At first, it might seem that we are ‘practising’ with patients. But, with experience, decision-making becomes more instinctual and less like practicing.”
The first step in developing the most appropriate treatment plan for a patient with dry eye disease (DED) is to correctly categorise the stage and type of DED the patient exhibits using the available technology. Then address the patient’s symptoms using the appropriate evidence-based therapies.
To make the right diagnosis, start with a complete DED examination. Think of this as analogous to diagnosing and managing glaucoma. Primary open-angle glaucoma is a diagnosis of exclusion: the patient’s intraocular pressure, nerve fibre layer, visual fields, cup-to-disc ratio, corneal hysteresis, anterior angles via gonioscopy, medical and family history, and other ancillary factors are all assessed. Treatment is based on whether aqueous production must be reduced or outflow must be improved. Over time, the patient is reassessed to determine if the patient is reaching target pressure or not and whether the visual field is stabilising. If not, the treatment plan must be made more aggressive. A similar process goes on in the care of patients with DED.
In 2007, a committee of optometrists, ophthalmologists, and scientists convened the DEWS. The aim of DEWS was to outline the information needed to fully diagnose and treat patients with DED. In the almost ten years since that effort, there have been significant advances in the field of DED. This article presents an update on how we approach the management of this disease where I practice, Vance Thompson Vision.
Multifactorial disease
Reports of the prevalence of DED vary in the literature but it is estimated to affect 30 to 40 million adults in the United States or about one out of every seven adults, and it is twice as common in women as men.1,2
DED, defined by the DEWS, is “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.”3 As many as 50% of patients with DED are asymptomatic, so it is important to screen all patients for DED.4 This can easily be done using one of the DED questionnaires that are readily available, such as the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire.5
It is also important to understand that there are two types of DED: evaporative and aqueous-deficient.6 Purely aqueous deficient DED is relatively rare, occurring in only about 10% of cases.7 Meibomian gland dysfunction (MGD) has been shown to be evident in about 86% of all DED cases, far outweighing purely aqueous deficient DED. Many patients have a combination of both conditions.7 Three forms of MGD have been recognised: hypersecretory, hyposecretory, and obstructive, with obstructive being the most common.
The incidence of MGD is thought to be rising because of an increase in incomplete blinking among patients.8 As the use of computers, handheld mobile devices, and smartphones increases, users tend not to blink as frequently or as completely as previously. The squeeze action that occurs with a blink is the force needed to release oil from the meibomian glands. With incomplete blinking, the oils in the glands become stagnant, hyperkeratinisation of the meibomian ducts occurs, and the glands start to atrophy, a phenomenon termed disuse atrophy.9 As this cycle occurs over long periods, further gland atrophy occurs, and, with the reduction of the lipid content of tears, evaporative DED develops.
This understanding is important to frame your approach to treating patients with DED; everyone must be assessed for DED. Much of the initial management will be based on the results of testing for osmolarity and inflammation. Most cases of DED are evaporative in nature due to deficiencies of the meibomian glands and, therefore, gland imaging is a crucial component of assessment.
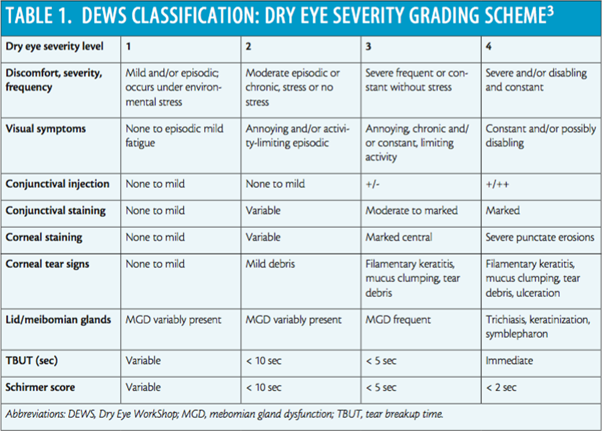
Inflammatory cascade
When we delve into what is happening in the inflammatory process at the cellular level, we find a cyclic cascade of events occurring. Intercellular adhesion molecule 1 (ICAM-1) is a protein found in the epithelial and endothelial cells of ocular tissues. ICAM-1 is located in excess in patients with DED. Adhesion molecules aid in recruiting other mediators toward sites needing attention.
In this case, ICAM-1 is sending signals for other inflammatory T cells to flock to its site to promote inflammation. A receptor on inflammatory T cells called lymphocyte function-associated antigen 1, or LFA-1, adheres to ICAM-1, activating the T cells and releasing cytokines that further increase ICAM-1 expression, leading to the continuation of the cycle of inflammation (Figure 1).10
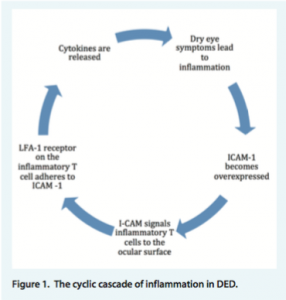
Grading DED
One of the products of the DEWS effort was a schema that helps practitioners to grade the level of severity of DED (Table 1). What is nice about this classification system is that it directly corresponds to both subjective and objective testing that eye care providers routinely utilise in clinical practice.
Two factors that this system does not include, however, are osmolarity (per the TearLab test) and inflammation (per the InflammaDry test), both of which are included in the DEWS definition of DED. In modern practices, osmolarity can be assessed using the TearLab Osmolarity Test (TearLab), and inflammation can be assessed with the InflammaDry test (Rapid Pathogen Screening). With a further understanding of the inflammatory element of DED, measurement of inflammation is crucial for the success of treatment and management. Because imaging devices that perform meibography, such as the LipiView II (TearScience) and Oculus Keratograph systems, are now available, this information can also be assessed and used to determine the proper course of action.
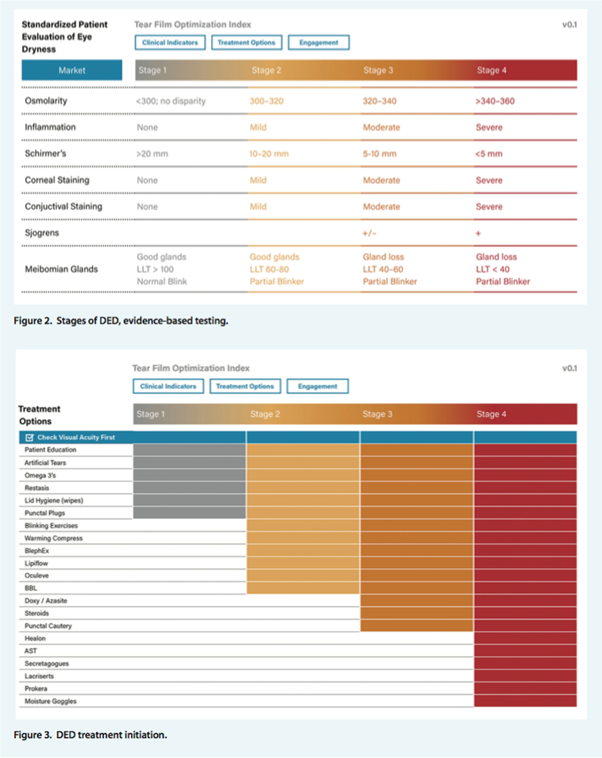
A better nomogram
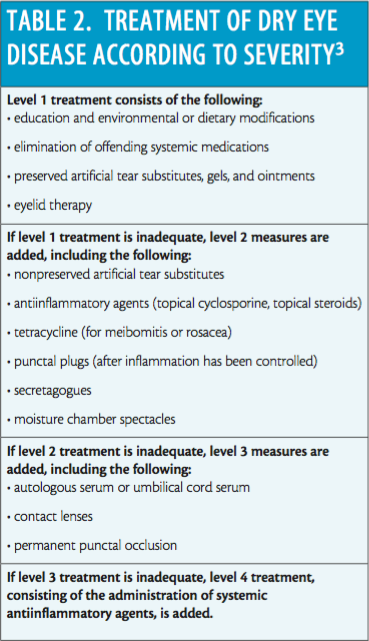
The guidelines for managing DED based on the 2007 DEWS findings are now almost ten years old.11 The DEWS treatment recommendations are stratified according to the severity of the disease (Table 2).
At Vance Thompson Vision, we have been diligently working on developing a nomogram to better classify the diagnostic levels of DED (based on severity and progression), and an algorithm tool for eye care providers. This tool would guide treatment decisions taking into consideration the diagnostic technologies now available and the treatments that might not have been available at the time of the DEWS report almost a decade ago.12
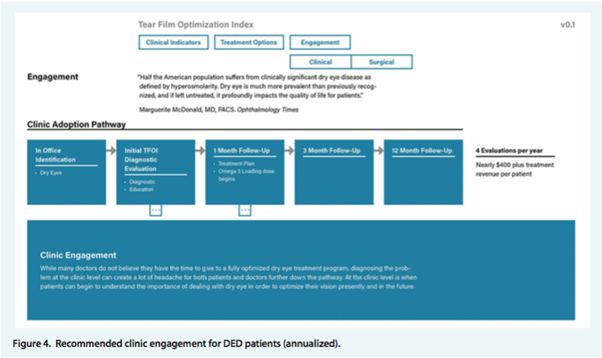
This nomogram includes a three-part process: clinical indicators, treatment options, and engagement (Figures 2-4). The first part (clinical indicators) includes the structured and standardised testing needed to diagnose patients into the correct stage of DED. The second part (treatment options) allows the clinician to better manage the patient by instituting the proper treatment regimen and the corresponding diagnostic tests.
Finally, the third part (engagement) helps set the standard for patients returning for follow-up care. (There can be nearly $400 in additional revenue annually when patients return for their scheduled DED follow-up appointments. In addition, incorporating a DED retail centre into the practice can be another significant source of revenue).
Conclusion
DED is a long-term, chronic disease that affects tens of millions of people in the United States, compared to about 2 million suspected glaucoma patients in this country.13 DED can disrupt the quality of life for many, and it is estimated that people spend up to $2 billion dollars annually on DED products and treatments.14
Recognising signs and symptoms early and educating patients on the root causes of their DED problems is critical to increasing patient compliance with your selected treatment plan. Specifically targeting the causes of each patient’s DED with products and treatments designed to improve function will make a difference in your patient’s well-being and quality of life.
DED is multifactorial, but it is defined by abnormal osmolarity and inflammation. If you incorporate new testing modalities into your practice and follow a systematic protocol for the management of the disease, hopefully your decision-making will be less like 'practising medicine' on your patients. You can potentially make your patients and yourself much happier.
About Jason Schmit, OD
In private practice specialising in oculoplastic, aesthetics, cataract, and refractive clinical care at Vance Thompson Vision in Sioux Falls, South Dakota.
President of ConceptualEyes, an ophthalmic consulting company specialising in improving operational excellence and overall practice growth.
References
- Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806.
2. Howden LM, Meyer JA. Age and Sex Composition: 2010. United States Census Bureau. May 2011. http://www. census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed May 4, 2016.
3. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92.
4. Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161-166.
5. Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204-1210.
6. American Academy of Ophthalmology. Dry Eye Syndrome PPP – 2013. http://www.aao.org/preferred-practice- pattern/dry-eye-syndrome-ppp–2013. Accessed May 4, 2016.
7. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
8. Blackie CA, Korb DR. Non-obvious meibomian gland disease. Optometric Management. June 2010.
9. Jester JV, Parfitt GJ, Brown DJ. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 2015;15 Suppl 1:156.
10. Cunningham KN. Overexpression of ICAM-1 in dry eye disease. Shire ClinTopics. S11568, 4-5.
11. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):163-178.
12. Schmit J, Jensen M. Internal research at Vance Thompson Vision. 2016.
13. American Optometric Association. Glaucoma FAQs. www.aoa.org/patients-and-public/eye-and-vision- problems/glossary-of-eye-and-vision-conditions/glaucoma/glaucom-faq?sso+y.
14. Cunningham D, Epstein A, Nichols K, Whitley W. Advancing the treatment of dry eye disease. Advanced Ocular Care. March 1, 2016.

 Back to Blogs
Back to Blogs